Establishing a national HTA program for medical devices in Italy: Overhauling a fragmented system to ensure value and equal access to new medical technologies - ScienceDirect

How can a joint European health technology assessment provide an 'additional benefit' over the current standard of national assessments? | Health Economics Review | Full Text

Shaping a research agenda to ensure a successful European health technology assessment: insights generated during the inaugural convention of the European Access Academy | Health Economics Review | Full Text
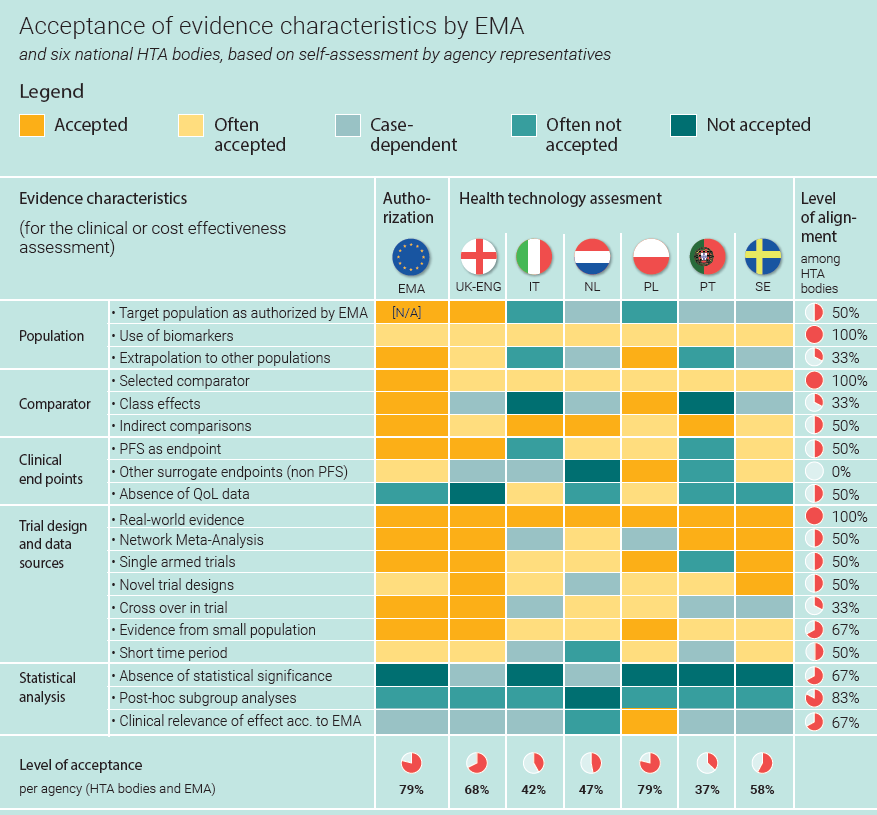
Europe's patchwork of evidence requirements is an important factor in delayed patient access - Consultancy in Healthcare and Life Sciences | Vintura Consultancy
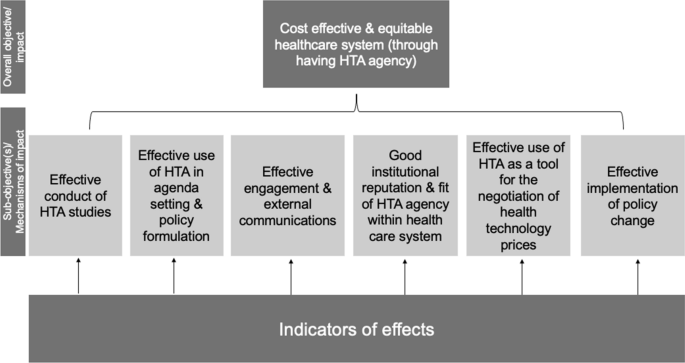
Assessing the performance of health technology assessment (HTA) agencies: developing a multi-country, multi-stakeholder, and multi-dimensional framework to explore mechanisms of impact | Cost Effectiveness and Resource Allocation | Full Text

Correspondence analysis biplot illustrating the relative associations... | Download Scientific Diagram